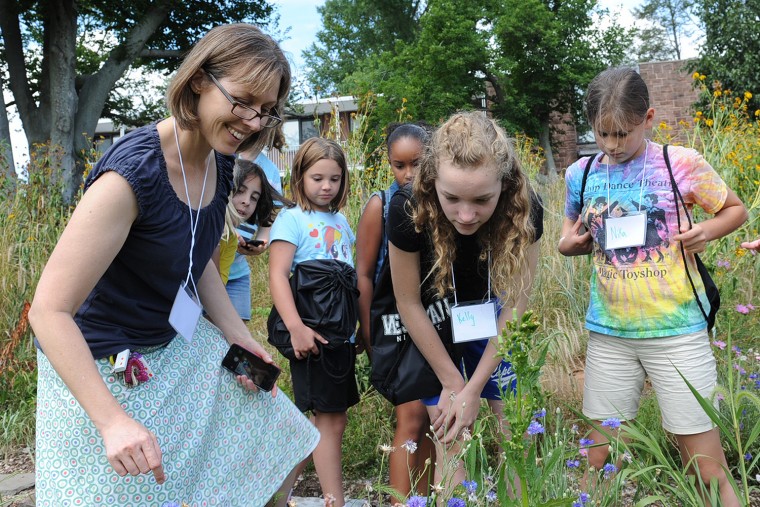From Nov. 9-12, two faculty members and five students from the physics and chemistry departments, attended the Annual Biomedical Research Conference for Minority Students in Tampa, Fla. Candice Etson, assistant professor of physics, and Erika Taylor, associate professor of chemistry, were joined by McNair Scholars Luz Mendez ’17, Tatianna Pryce ’17, Stacy Uchendu ’17 and Hanna Morales ’17; and Wesleyan Mathematics and Science (WesMaSS) Scholar Helen Karimi ’19. Students observed other research being performed around the nation by students who are members of underrepresented groups in Science, Technology, Engineering and Mathematics (STEM). In addition, the Wesleyan students presented their own research and Morales and Karimi…
Erika Taylor, associate professor of chemistry, is a co-author of a paper titled, “Methyl transfer by substrate signaling from a knotted protein fold,” published in the August 2016 issue of the Nature Structural & Molecular Biology newsletter. The paper describes the protein TrmD, an enzyme that catalyzes tRNA modification, but unlike most proteins, TrmD has an "interesting knotted configuration, which is not common," Taylor said. The paper demonstrates that even in proteins with knotted configurations, the internal protein movements and dynamics are important for binding, signaling and catalysis. "This is exciting because one might expect knotted proteins to be more static in…
The Green Street Teaching and Learning Center hosted a Girls in Science Camp Aug. 3-7. Wesleyan faculty members Ruth Johnson, assistant professor of biology (pictured third from left); Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies (pictured at far right); Chris Othon, assistant professor of physics (pictured at left), along with three undergraduate students, worked with the campers on various experiments. Sara MacSorley, director of the GSTLC (second from left), coordinated the activities. Johnson led the campers on a bug hunt through Wesleyan's West College Courtyard garden. There, the girls observed insects while considering insect diets and insect life-cycles. The girls…
Science in Society major Stacy Uchendu '17 is participating in Wesleyan's Ronald E. McNair Post Program, which assists students from under-represented groups in preparing for, entering and progressing successfully through post graduate education. Stacy is researching second generation biofuels as a McNair Scholar.
On June 15, Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, received a grant from the National Institute of Allergy and Infectious Diseases (part of the National Institutes of Health) to support her research on “Inhibition of (the enzyme) HeptosyltransferaseI for the treatment of Gram-negative bacterial infection.” Gram-Negative bacteria include things like E. coli, Salmonella, and V. cholerae (the cause of Cholera) that are common causes of food-bourne illnesses. The grant, worth $492,000 will enable her to engage multiple graduate and undergraduate students in the proposed work through June 2018. Preliminary results for this project were obtained…
Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, has co-authored a paper published in FEBS Letters, an international journal established for the rapid publication of final short reports in the fields of molecular biosciences. The paper, which is an expansion of her lab’s work on the enzyme Heptosyltransferase I, is titled "Cloning and Characterization of the Escherichia coli Heptosyltransferase III: Exploring Substrate Specificity in Lipopolysaccharide Core Biosynthesis," The paper is co-authored by her former graduate student Jagadesh Mudapaka. FEBS Letters is published by Elsevier on behalf of the Federation of European Biochemical Societies. Taylor also is the co-author of “Improving Alternate Lignin Catabolite Utilization of LigAB…
(more…)
Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, delivered the keynote address at the 16th Annual Undergraduate Research Symposium, hosted by the School of Natural Sciences of Fairleigh Dickinson University on April 25. Taylor spoke on "Alternative Energy Sources: Enzymology That Is Essential for Making Lignin." At Wesleyan, Taylor is exploring the enzymology that is essential for making Lignin a viable biomass source for production of energy and as a commodity chemical feedstock.
Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, and chemistry graduate student Kevin Barry, are the co-authors of an article titled "Characterizing the Promiscuity of LigAB, a Lignin Catabolite Degrading Extradiol Dioxygenase from Sphingomonas paucimobilis SYK-6," published in Biochemistry. This article is part of their effort to enable the utilization of lignin, the world’s second most abundant natural polymer, as a carbon source for the production of bioenergy and chemical feedstocks. An abstract will soon be available online here.
Two faculty members and two graduate students co-authored a paper published in the July 18 edition of the academic journal, Biochemistry. Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies; Manju Hingorani, professor of molecular biology and biochemistry; chemistry graduate student Daniel Czyzyk; and molecular biology and biochemistry graduate student Shreya Sawant wrote the paper, "Escherichia coli Heptosyltransferase I: Investigation of Protein Dynamics of a GT-B Structural Enzyme." It appears online here. Biochemistry is a publication of the American Chemical Society.
During extended space travel, astronauts may experience dramatic health consequences, such as anemia, due to reduced gravity and exposure to space radiation. To help combat the adverse effects of space ailments, two scientists at Wesleyan are developing new molecules that enhance cells' ability to tolerate large swings in pressure, fluid redistribution, temperature and radiation exposure. Christina Othon, assistant professor of physics, and Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, received a $20,000 seed grant from NASA's Biological and Physical Research Enterprise to work on the project titled "Osmoregulation for Microgravity Environments." The scientists are taking inspiration from…
Erika Taylor, assistant professor of chemistry, assistant professor of environmental studies, is the co-author of "Lipopolysaccharide Biosynthesis without the Lipids: Recognition Promiscuity of Escherichia coli, Heptosyltransferase I," published by the American Chemical Society in Nov. 2011. The abstract is available here.