Research conducted by a Wesleyan professor is part of a new space show at the American Museum of Natural History. Martha Gilmore, George I. Seney Professor of Geology and professor of Earth and environmental sciences, worked over the past year developing content for the new Hayden Planetarium Space Show Worlds Beyond Earth. The show opened on Jan. 21 as part of the museum's 150th anniversary celebration. "It’s amazing," Gilmore says. "The images that you see are all realistic. We even contacted some of the engineers for the Magellan spacecraft in order to understand exactly how the spacecraft imaged Venus in…
Wesleyan faculty frequently publish articles based on their scholarship in The Conversation US, a nonprofit news organization with the tagline, “Academic rigor, journalistic flair.” In a new article, John Monroe Van Vleck Professor of Astronomy Bill Herbst and Assistant Professor of Earth and Environmental Sciences James Greenwood write about the model they've proposed for how the most common kind of meteorites form—a mystery that has dogged scientists for decades. The tell-tale clue to how meteorites were made, at the birth of the solar system April 26, 1803 was an unusual day in the small town of L’Aigle in Normandy, France – it…
A paper by John Monroe Van Vleck Professor of Astronomy William Herbst and Assistant Professor of Earth and Environmental Sciences James Greenwood will be published in the September 2019 issue of Icarus, published by Elsevier. The paper is available online. The paper, titled "Radiative Heating Model for Chondrule and Chondrite Formation," presents a new theory of how chondrules and chondrites (the most common meteorites) formed. It suggests a new approach to thinking about these rocks that populate the meteorite collections on Earth. It includes both theory and experiments (completed in Greenwood's lab in Exley Science Center). These laboratory experiments demonstrate that porphyritic olivine…
Several Wesleyan students, faculty, and alumni attended the 50th Lunar and Planetary Science Conference (LPSC) March 18-22 in The Woodlands, Texas. Members of the Wesleyan Planetary Sciences Group presented their research on a range of planetary bodies. This annual conference brings together international specialists in petrology, geochemistry, geophysics, geology, and astronomy to present the latest results of research in planetary science. Earth and environmental studies major Emmy Hughes '20 presented a poster titled “Observations of Transverse Aeolian Ridges in Digital Terrain Models" during a session on “Planetary Aeolian Processes.” Earth and environmental science graduate student Reid Perkins MA '19 presented a…
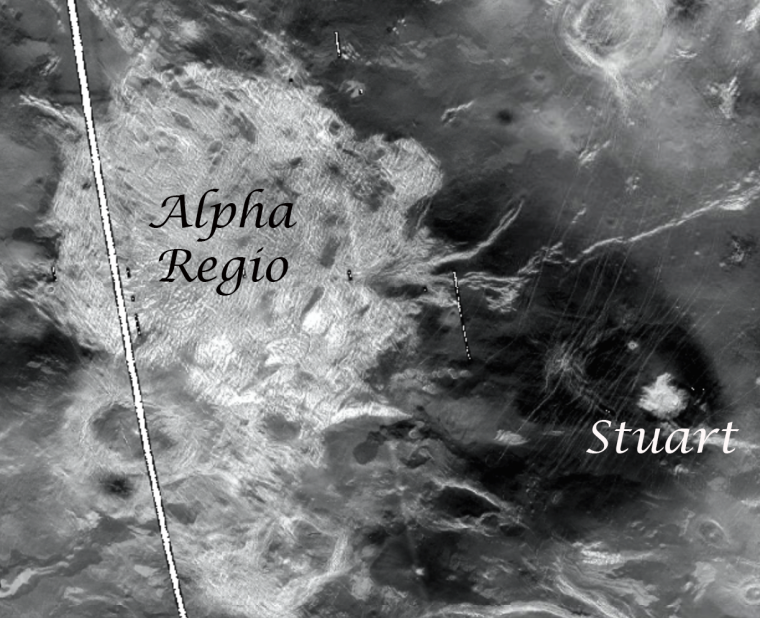
Like planet Earth, the geology of Venus is diverse; consisting of areas of flat plains and deformed, mountain-like terrains called tesserae. And like Earth, Mars, and the Moon, Venus is checkered with hundreds of craters. “What's odd about Venus's craters, is that craters we do see are relatively young, indicating the surface of Venus has been covered by planet-wide volcanic flows," says Martha "Marty" Gilmore, George I. Seney Professor of Geology, professor of earth and environmental sciences. “The tesserae are the only terrains older than these volcanic flows and thus our only hope at accessing rocks from the first billion…
By using a newly acquired electron microscope, the E&ES 368 Meteorites and Cosmochemistry class was able to classify a meteorite discovered in Morocco. "We were able to determine that it was an H4 ordinary chondrite, and the chemical information being collected today will be used to document these findings and submit this meteorite to the Meteorite Nomenclature Committee of the Meteoritical Society for official classification," said class instructor Jim Greenwood, assistant professor of earth and environmental sciences. Wesleyan acquired the field-emission scanning electron microscope (FE-SEM) with support from a $202,300 National Science Foundation grant awarded in August 2017. Greenwood and Michelle Personick,…
Two faculty members and three students have been awarded grants in the latest call for proposals from NASA's Connecticut Space Grant Consortium. Jim Greenwood, assistant professor of earth and environmental sciences, and Bill Herbst, the John Monroe Van Vleck Professor of Astronomy, professor of integrative sciences, were awarded $8,000 for a Faculty Collaboration Grant titled “Chondrule Formation Experiments.” This is to run high-temperature experiments on material that makes up meteorites in order to test a hypothesis that they put forward in a recent paper in Icarus this year. Seth Redfield, associate professor of astronomy, associate professor of integrative sciences, was awarded $1,500 for a STEM Education…
Bill Herbst, the John Monroe Van Vleck Professor of Astronomy, and James Greenwood, assistant professor of earth and environmental sciences, co-authored an article published in the planetary science journal Icarus. Their article, “A New Mechanism for Chondrule Formation: Radiative Heating by Hot Planetesimals” grew out of research seminars from the recently introduced Planetary Science graduate concentration and minor at Wesleyan. Their work focused on chondrules, or tiny spheres of molten rock that permeate primitive meteorites and date to very close to the beginning of the solar system. For decades, the existence of chondrules has puzzled astrophysicists and cosmochemists as no…
Jim Greenwood, assistant professor of earth and environmental sciences, was awarded a Faculty Seed Research Grant from the Connecticut Space Grant Consortium, supported by NASA. The honor comes with a $6,000 award. Greenwood will use the grant to support his research on “D/H of ‘Dry’ Extraterrestrial Materials.” Understanding the distribution, delivery, and processing of volatiles in the solar system is of fundamental interest to planetary science. Volatiles influence a number of important properties of planetary bodies, such as the cooling, differentiation, volcanism, tectonism, climate, hydrosphere/atmospheres and especially habitability. Greenwood will use the award to develop a new state-of-the-art inlet system for the measurement…
As a recent recipient of an undergraduate research fellowship, Jack Singer '15 is spending his summer at Wesleyan studying the geochemical evolution of the moon. The fellowship, supported by the Connecticut Space Grant College Consortium, comes with a $5,000 award. Grantees are expected to work on research related to space/aerospace science or engineering under the guidance of a faculty member or a mentor from industry. For the next three months, Singer will work on various research projects with his advisor James Greenwood, assistant professor of earth and environmental science. Singer will first prepare a fragmented lunar sample (Apollo 12035,76) for…
Assistant Professor of Earth and Environmental Sciences James “Jim” Greenwood has received a $331,000 grant from NASA to support his research on the moon’s water. His proposed research, tracking water in rock samples brought back by the Apollo missions, will “take a giant leap towards solving one of the most important questions in planetary science – whether the Moon is wet or dry,” Greenwood said. “We’ll be studying pockets of glass trapped in early and late-crystallizing minerals in lunar mare basalt samples,” Greenwood said. “We will measure water and other volatile elements in these trapped melt pockets to reconstruct the…
James "Jim" Greenwood, assistant professor of earth and environmental sciences, and four colleagues have published a paper that casts doubt on the theory of abundant water on the moon while simultaneously boosting theories around the creation of the moon, several billion years ago. The paper, “The Lunar Apatite Paradox,” published March 20 in the prestigious journal Science, stems from work involving the mineral apatite, the most abundant phosphate in the solar system. (Along with its presence on planets, it’s found in teeth and bones.) Initial work on the lunar rocks brought back to Earth by the Apollo missions indicated that…